We actively engage with visionary biotech companies driving progress through key clinical milestones.
SJA, a division of Finalis Securities LLC, Member FINRA / SIPC, works closely with promising biotechnology companies advancing preclinical and clinical programs through transformative inflection points. We welcome the opportunity to connect and learn more about your company. Please click the link to schedule a conversation.
Biologics: mAb (Monoclonal Antibodies) / Radiopharmaceuticals

Company Overview
ImaginAb is focused on non-invasive, whole-body, in vivo PET imaging of CD8 T cells. The company’s goal is to visualize the immune system’s response to therapy, and thus change the way in which cancer patients are treated by tailoring immuno-oncology treatments for best patient outcomes.
Market Opportunity: High Unmet Need in Immuno-Oncology
Immuno-oncology (I-O) is an exciting, growing, and effective field. However, establishing the right treatment for each patient has been challenging. Moreover, once treatment has started, it can take months to determine whether the patient is responding or progressing. Costs are estimated to be over $150,000 per annum per patient, with response rates typically around 20-30%. Current methods used to address these challenges (e.g., biopsy/liquid biopsy, biomarkers, imaging, etc.) are ineffective.
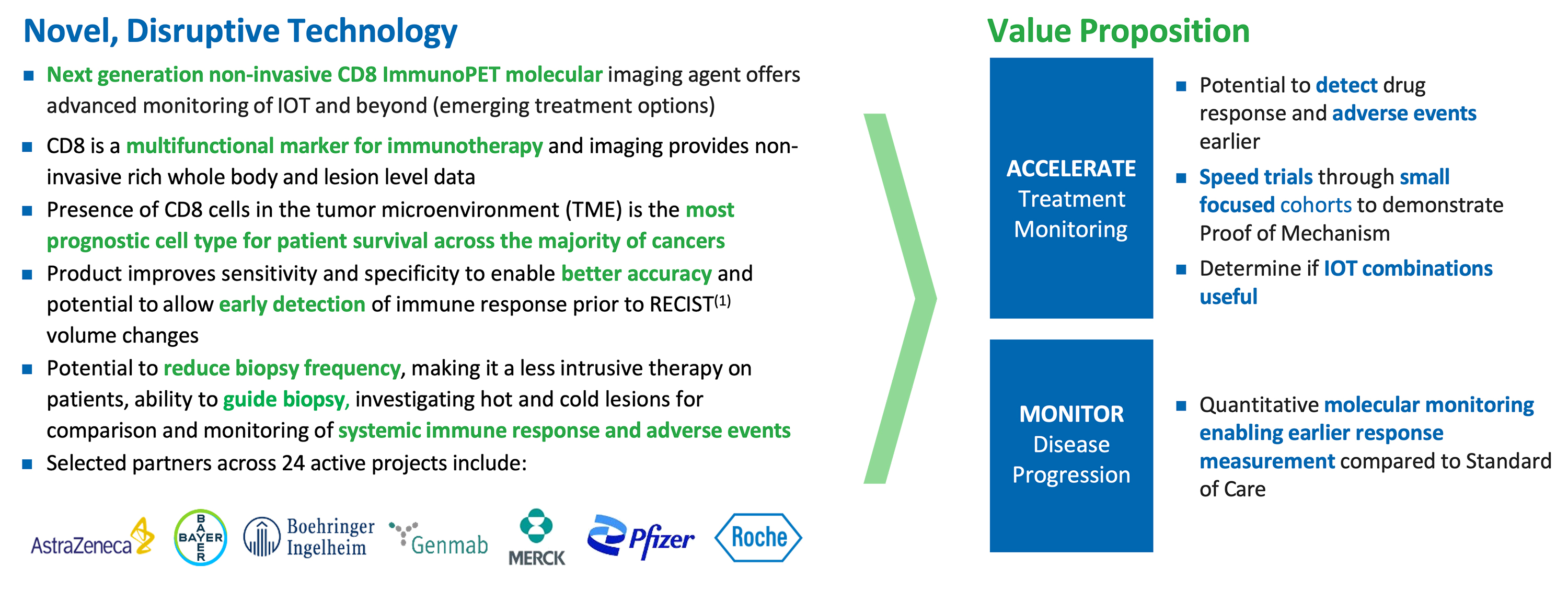
ImaginAb's Solution: CD8 T Cell ImmunoPET
ImaginAb using its proprietary technology platform, has developed a humanized engineered antibody around half the size of an intact antibody (“minibody”) designed to have an affinity for human CD8 T cells, and an IP protected 89Zr-minibody platform for non-invasive imaging. 89Zr half-life of 3.2 days enables centralized distribution (e.g., one manufacturing site for North America). The ImmunoPET agent is infused in the body and an image is taken using a standard PET camera. This is similar to an F-18 FDG scan. However, instead of imaging cancer, ImaginAb’s ImmunoPET is imaging the CD8 T cells (i.e., the immune system response).
CD8 ImmunoPET Imaging Product Overview

ImmunoGenesis brings a clear and singular focus to the complex realm of cancer treatment. The company is developing science-driven immunotherapies specifically designed to treat tumors lacking activated T cells or having other immune resistance mechanisms. Because these “cold” tumors are resistant to existing immunotherapy, our team is striving to create sophisticated therapies that target key mechanisms of immune resistance. ImmunoGenesis approaches treatment for cold tumors through a new lens, re-envisioning treatment with a deliberate drug development strategy based in cold tumor pathology.
ImmunoGenesis’ novel PD-L1/PD-L2 dual-specific inhibitor is a platform molecule around which several promising treatments for cold tumors can be built. This is the first antibody to target PD-L2. This vision puts ImmunoGenesis at the forefront of scientific exploration. Built utilizing our platform, our lead asset was engineered with robust effector function. Another PD-L1/PD-L2 antibody from this platform is being developed as a tumor-selective delivery vehicle for our potent stimulator of interferon genes (STING) agonist.
Foundational to ImmunoGenesis’ vision is a first‑in‑class PD‑L1/PD‑L2 dual‑specific antibody. PD‑1 suppresses T‑cell activation through binding its ligands, PD‑L1 and PD‑L2. PD‑L2 is a critical regulator of human tumor immunity. Pairing it with the known action of the PD‑L1 inhibitor means the platform, at minimum, offers the same blocking function as a PD‑1 inhibitor. Building on that action, we are designing it with effector function, which allows the antibody to kill the immunosuppressive cells in the tumor microenvironment. Additionally, the antibody may also function as a tumor‑specific delivery vehicle and as the base for dual‑specific programs, expanding targeting options. For design and creation, we partnered with a novel antibody development company. Through trial and error and multiple rounds of affinity maturation, we developed a novel antibody in which both arms bind with clinically relevant affinities to both PD‑L1 and PD‑L2.
Scientific Foundation
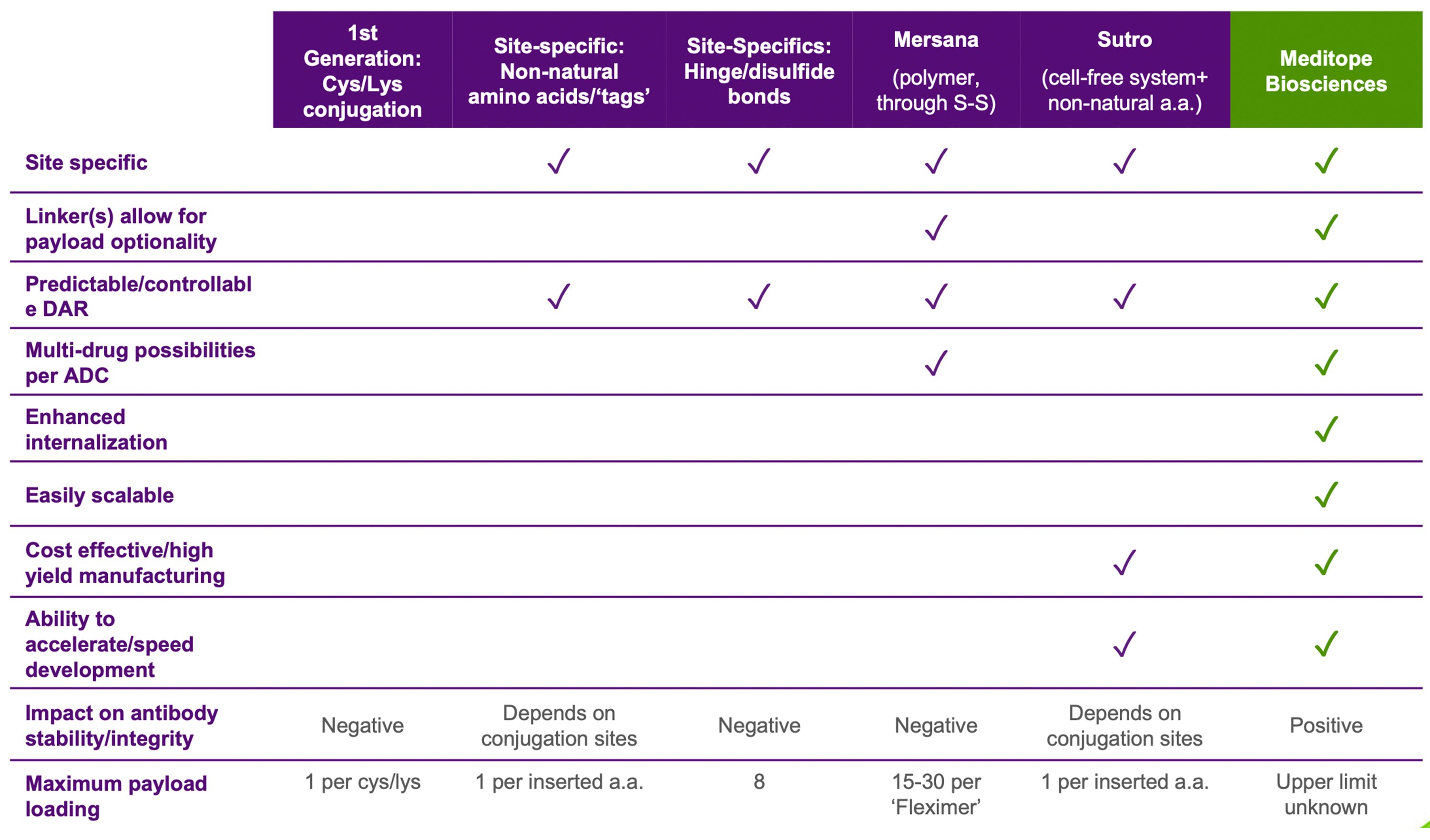
Meditope is a pre-clinical oncology company leveraging its proprietary platform technology which allows for the selective attachment of anti-cancer agents and biologicals to antibodies and other targeting moieties providing specific targeted delivery to the tumor. The company’s first indication will be a prostate tumor targeted cytokine fusion to selectively target prostate cancer cells with an otherwise toxic treatment. Meditope’s patent suite covers a broad-based diagnostic/therapy platform - a Lego®-like system that allows the coupling of a molecular payload (drug, radiotracer, marker) to any antibody. The binding site is engineered into a specific antibody and the binding is specific and controllable. The affinity-driven conjugation allows for more efficient and gentler payload attachment that can be made covalent for maximal stability or non-covalent for planned dissociation.
Enabled Antibodies
Specific amino acids of antibody may be switched out to enhance affinity of binding and amino acids are always chosen based on “rules” designed to preserve antibody integrity. Enablement retains normal antibody function (example: antigen recognition). Noncovalent and covalent binding is possible with single enablement (requires different peptides)
Superior Conjugation Technology
Gene Therapy

Gene therapy for the treatment of Atrial Fibrillation
Inomagen Therapeutics is an early-stage company dedicated to developing and commercializing a biological (gene) therapy to improve the treatment of atrial fibrillation (AF) by targeting the underlying cause of the disease. AF is the most common sustained heart rhythm disorder affecting 6M people in the U.S. and 33M worldwide. Those suffering with AF have a ~6x higher risk of stroke leading to 750,000 hospitalizations and 130,000 deaths globally. AF incidence increases significantly with age, making the disease an epidemic in developed countries.
Unfortunately, current AF treatments have suboptimal efficacy. Drugs to control heart rhythm have <50% efficacy and can cause life threatening arrhythmias and significant side effects. For drug refractory patients, cardiac ablation is moderately successful (70%) treating early stage (paroxysmal) AF, however, often requires repeat procedures. Most troubling is cardiac ablation’s 30-50% success treating more advanced stage (persistent) AF representing the majority of symptomatic, drug refractory AF patients. Despite best efforts, improvements in ablation catheters and energy sources have led to only modest increases in ablation success.
Inomagen is developing a biological (gene) therapy targeting >70% efficacy for all AF patients including under-treated patients with advanced stage (persistent) AF. With the support of significant grant funding (>$20M), RTI identified fundamental molecular mechanisms in the creation of the AF disease state and major trans-genes to selectively target these mechanisms. In proof-of-concept studies, Inomagen’s gene therapy technology demonstrated a marked decrease in AF in clinically relevant, large animal models.
GENE THERAPY HAS POTENTIAL TO SURPASS CARDIAC ABLATION AS THE AF TREATMENT OF CHOICE
- Inomagen’s therapy directly targets the molecular mechanisms underlying AF and has potential to be more effective than current standard ion-channel blockers and ablation
- Inomagen’s gene therapy approach attenuates both electrical remodeling (assessed in the Rapid Atrial Pacing, RAP, mode) as well as fibrosis (Heart Failure Model) in large animals
- Potential to substantially reduce mortality, improve quality of life, and control healthcare costs
- Avoids creating destructive ablative lesions of heart tissue which do not have the capacity to regrow, and can limit future AF treatment options
- Uses a novel trans-venous delivery approach making the procedure comparable to or simpler than current AF ablation
- Simultaneous electroporation in atrial region using a commercially available electrophysiology mapping catheter achieved excellent atrial gene transfer
If you would like to learn more about these promising technologies, please email Scott Jordan at scott@sjordanassociates.com